Our high containment biologics operations handle highly infectious and/or potent agents through a controlled manufacturing system through the use of BSL-3/BSL-2 infrastructure with a capability for closed system processing.
We currently have multiple processing suites and supporting infrastructure, including a dedicated process development suite and CDC Tier 1 select agents. For each biological agent introduced, aggressive assessment studies are performed to ensure there is no contamination.
Our analytical testing labs are furnished with appropriate equipment such as safety work benches for sample preparations, containment hoods and isolators, and separation from the normal lab areas and with limited access.
BioMARC complies with all applicable laws with respect to the environment, occupational health and safety, public health and safety, and waste disposal, and holds all current and applicable governmental licenses, approvals, permits and authorizations.
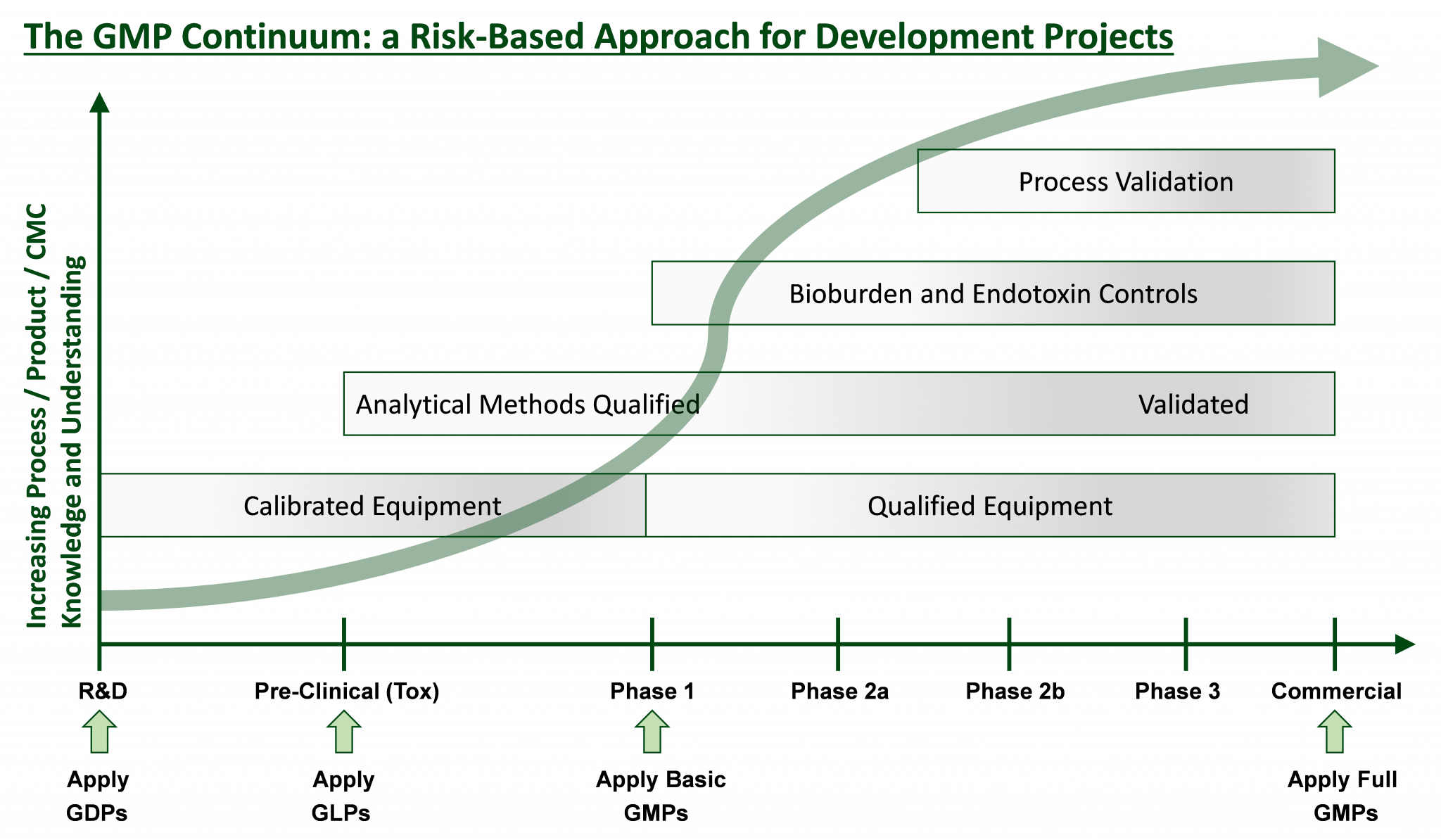
The graph below represents the appropriate regulatory framework applied at each stage of development, ensuring regulatory compliance and reasonable cost.